‘Heat’ describes the total thermal energy of a substance, while ‘temperature’ is a measure of the average kinetic energy of the molecules within a substance. An understanding of these topics is expected in the Primary FRCA.
Here on TeachMeAnaesthetics, we aim to cover the absolute key details of high yield exam topics, which can then be supplemented with further reading as needed.
In this article, we will discuss the key concepts of heat and temperature, and discuss the methods by which thermal energy is transferred in the operating theatre.
Definitions
Heat is a slightly abstract concept, which is defined by the total energy of molecular motion within a substance. This is affected by both the mass of the substance and the kinetic energy of its constituent molecules. A big, cold substance can still have more heat energy than a small, hot substance.
Temperature is more straightforward, and is a measure of the average kinetic energy of its constituent molecules.
Confusing? Perhaps. To simplify, E-LFH has a nice analogy which we have borrowed and altered here in the interests of clarity:
“An iceberg, despite having a lower temperature than a human, has greater overall heat energy due to its significantly greater mass. However, on contact, heat energy will transfer from the human to the iceberg. This demonstrates how temperature is defined by the likelihood for a substance to transfer heat energy to another.
Similarly, the human body has a higher temperature than the operating theatre. Therefore, despite having less heat energy in total, the human body releases thermal energy into the operating theatre.”
Specific Heat Capacity
As with large parts of the physics curriculum, we have more definitions to work through. Heat capacity is the amount of energy required to raise the temperature of an object by 1 Kelvin. Specific heat capacity is…more specific…and is the amount of energy required to raise the temperature of 1 kg of a substance by 1 Kelvin.
The equation is as follows:
Equation 1: c = ΔQ/m.ΔT
where c = specific heat capacity, ΔQ is the amount of heat energy (kJ), m is the mass (kg) and ΔT is the change in temperature.
If asked ‘how much energy is required to raise the temperature of substance X, mass Y, by Z degrees Kelvin?’, the equation can be reworked as follows:
Equation 2: ΔQ = c.m.ΔT
Mechanisms of Heat Loss
“So, why do patients get cold in the operating theatre?” is a very predictable physics viva question, and one that would be expected to be answered thoroughly. The answer has two parts to it; firstly, how energy is transferred, and secondly, why this happens in the operating theatre:
Energy Transfer
The mechanisms (and estimated %contribution) of energy loss in the operating theatres are as follows:
- Radiation (40%): Energy loss through infrared radiation.
- Convection (30%): Energy loss due to air currents which receive thermal energy and then migrate, maintaining a gradient for further energy transfer.
- Evaporation (15%): Energy loss due to the latent heat of vaporisation.
- Respiratory losses (10%): Further evaporative heat loss
- Conduction (5%): Direct energy transfer onto surrounding surfaces e.g. operating table
Unhelpfully, the %contributions vary according to different sources. We took ours from an excellent BJA article on the topic.
Why in the Operating Theatre?
Both regional and general anaesthesia exacerbate the situation, and predispose the patient to temperature loss.
- Anaesthetic effects: Paralysis prevents shivering, reduction in muscle tone reduces heat generation, vasodilatory effect of agents increases energy loss, reduction in basal metabolic rate.
- Physical effects: Exposure of the skin surface, exposure of the abdomen (laparotomy), inhibition of behaviour change (move to warm environment)
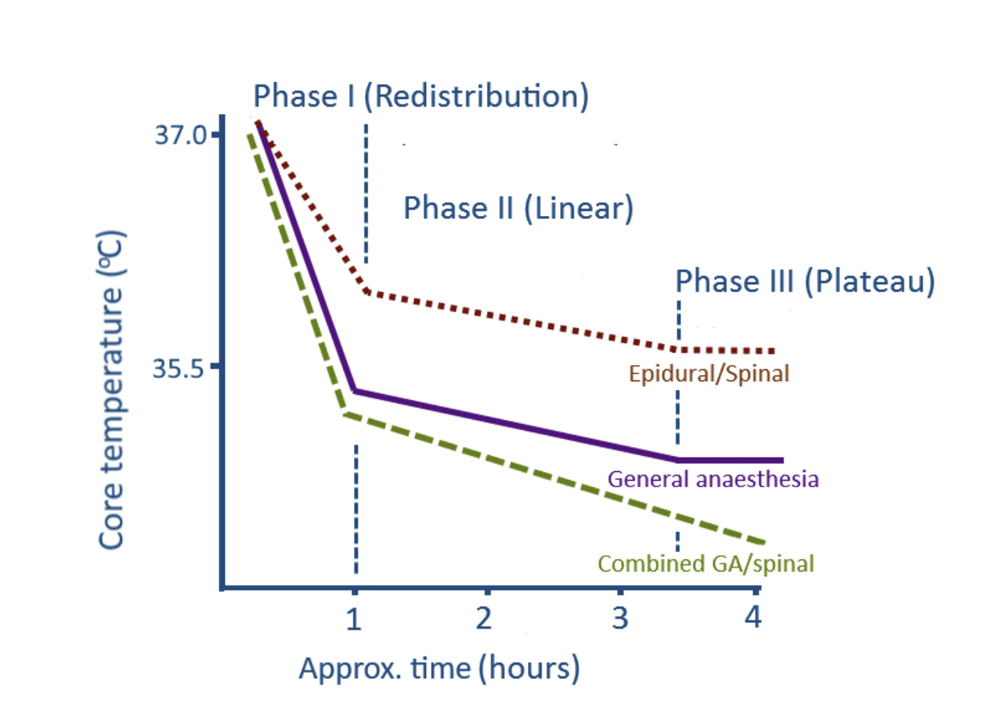
Stages of Heat Loss
There are three stages of heat loss described in the literature:
1 – Redistribution: A sharp decline in body temperature due to vasodilation and movement of blood to the peripheries
2 – Linear phase: A more gradual decline until a new set-point is reached
3 – Equilibrium: Temperature is maintained at this new level
Suggested Reading
BJA article: https://academic.oup.com/bjaed/article/8/3/104/293364
E-LFH: http://portal.e-lfh.org.uk/Component/Details/417479
Pages 40, 50-53: Physics, Pharmacology and Physiology for Anaesthetists. Key Concepts for the FRCA. 2nd edition. Cross and Plunkett. 2014.