Given the daily use of gases by anaesthetists, it follows that an understanding of how gases behave, as dictated by the gas laws, is necessary for the Primary FRCA.
Here on TeachMeAnaesthetics, we aim to cover the absolute key details of high yield exam topics, which can then be supplemented with further reading as needed.
In this article, we will discuss the gas laws, and demonstrate their clinical relevance where appropriate.
The Ideal Gas
Firstly, it is important to acknowledge that the gas laws only truly apply to the ‘ideal gas‘. Unhelpfully, the ideal gas does not exist. However, if it did, it would have the following characteristics:
- All collisions are elastic and frictionless
- The molecules move in random directions at a distribution of speeds
- There are no attractive or repulsive forces between gas molecules
- The molecules of gas are small, hard spheres
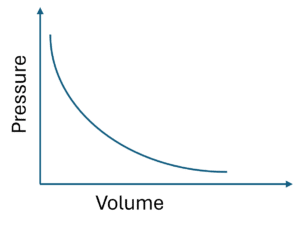
Boyle’s Law
The first gas law is Boyle’s law, which states:
“At a constant temperature in a sealed container, the volume of a gas is inversely proportional to its pressure.”
Simply put, as pressure increases, the volume decreases, and vice-versa.
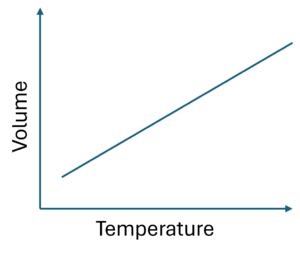
Charles’ Law
The second gas law is Charles’ law, which states:
“At a constant pressure, the volume of a gas is directly proportional to its temperature.”
Effectively, Charles’ law describes a linear relationship between volume and temperature.
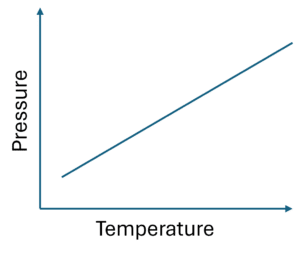
Gay-Lussac’s Law
The third gas law is Gay-Lussac’s law. This one says:
“At a constant volume, pressure and temperature are directly proportional.”
Again, this describes another linear relationship; just this time it is pressure and temperature.
The Ideal Gas Law
Through mathematical manipulation (available here on Wikipedia for the mathematically inclined), the above three laws can be combined into the ideal gas equation. This one states:
PV = nRT
where P = pressure (Pascals), V = volume (m3), n = no. of moles of the gas, R = molar gas constant, and T = temperature (Kelvin)
This is sometimes also written as:
P1V1/T1= P2V2/T2
Dalton’s Law
Dalton’s law is relatively simple, and states that the pressure of a gas is the sum of the partial pressures of each of its constituent gases.
This is of particular relevance when discussing the effects of altitude on the delivery of volatile agents, but an explanation of this somewhat confusing topic is beyond the scope of this article.
A good discussion of the topic can be found in Chapter 77 of The Primary FRCA structured oral examination Study Guide 1, 2nd edition, Wijayasiri and McCombe, 2016.
Henry’s Law
Henry’s law states that the partial pressure of a gas above a liquid is directly proportional to the amount of gas dissolved within said liquid, at a given temperature.
This explains why bubbles exit fizzy drinks when the cap is removed. With the cap shut and the system closed, the partial pressure of carbon dioxide is high and is in equilibrium with dissolved carbon dioxide. Upon removal of the cap, the partial pressure of carbon dioxide rapidly drops, thus shifting the equilibrium and allowing bubbles to exit from solution.
Suggested Reading
Chapter 53. The Primary FRCA structured oral examination Study Guide 1. 2nd edition. Wijayasiri and McCombe. 2016.
Pages 34-35. Physics, Pharmacology and Physiology for Anaesthetists. Key Concepts for the FRCA. 2nd edition. Cross and Plunkett. 2014.
Sections 5.12, 5.13 and 5.16. Graphic anaesthesia: Essential diagrams, equations and tables for anaesthesia. 2nd edition. Hooper, Nickells, Payne, Pearson and Walton. 2023.