Local anaesthetics play a key role in modern anaesthetic practice, and therefore a detailed knowledge of them is expected for the Primary FRCA examinations.
Here on TeachMeAnaesthetics, we aim to cover the absolute key details of high yield exam topics, which can then be supplemented with further reading as needed.
In this article, we will provide a brief overview of the classes, mechanism of action and physiochemistry of these commonly used drugs.
Classes of Local Anaesthetics
Local anaesthetics consist of an aromatic ring and an amine side chain, which are connected by either an ester or an amide group. Therefore, local anaesthetics are classified into the esters and the amides.
Esters
The ester local anaesthetics include cocaine, amethocaine and procaine (CAP). Compared to amides, they are short acting due to their rapid metabolism by esterases within the plasma.
They also have a higher incidence of hypersensitivity reactions compared to amides due to the generation of PABA (p-aminobenzoic acid) during their metabolism.
Amides
The amides are arguably used more commonly than esters, and can be easily identified by the ‘i’ within their name followed by the -caine suffix e.g. lidocaine, prilocaine, bupivacaine.
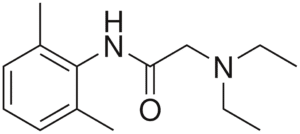
Figure 2
Lidocaine, with the amide (NHCO) group visible in the middle of the compound
Mechanism of Action
Local anaesthetics act by interfering with action potential generation. In the neuronal action potential, depolarisation occurs due to the opening of voltage-gated sodium channels, with subsequent influx of sodium from the extracellular space to the intracellular space.
In short, local anaesthetics exert their effect by crossing the cell membrane, becoming ionised, and blocking sodium channels from inside the cell. By blocking sodium channels, sodium influx is reduced and the affected neurone becomes unable to conduct an action potential.
As the cell membrane consists of a phospholipid bilayer, the local anaesthetic must be in its unionised form to enter the cell and exert its effect. All local anaesthetics are weak bases, and so are ionised when local pH < their pKa. Therefore, we can see that in the presence of acidosis, a greater proportion of the local anaesthetic will be ionised, and will therefore be less able to exert its effect.
This explains why local anaesthetics are less effective when used in infected tissue e.g. an abscess.
Pharmacological characteristics
Each local anaesthetic agent varies in their protein binding, lipid solubility and pKa. Importantly, these properties also dictate how a local anaesthetic behaves.
Protein binding
The protein binding of a local anaesthetic dictates its duration of action. A more heavily protein bound agent (bupivacaine, 95%) has a much longer duration of action than a less heavily protein bound agent (prilocaine, 55%).
Lipid solubility
The relative lipid solubility dictates the potency, where potency describes the dose of an agent that is required to provide a given effect. A more potent agent requires a lower dose for a given effect as compared to a less potent agent.
Highly lipid soluble agents such as bupivacaine need a lower dose than less lipid soluble agents such as prilocaine.
pKa
As a reminder, the pKa of a drug is the pH at which the drug exists in its unionised and ionised forms in equal proportions. As we saw above, a local anaesthetic needs to be in its unionised form to cross the cell membrane and become active.
The pKa dictates the time of onset. A drug which is highly ionised (where pH is much lower than pKa) will have a smaller active fraction, and so will take longer to cross the cell membrane in sufficient amounts to generate an effect.
Therefore, drugs with a higher pKa (bupivacaine, 8.1) have a slower onset than those with a lower pKa (prilocaine, 7.7) at physiological pH.
Clinical Relevance
Epidural top-ups
Epidural top-ups are frequently used in the obstetric setting to turn an epidural from an analgesic technique into a technique that provides sufficient anaesthesia for an emergent surgical procedure.
In trusts that use lidocaine for their top-ups, some centres will add bicarbonate to their local anaesthetic mixture. The effect of this is to raise the pH of the solution, such that pH begins to approach the pK of lidocaine, and therefore a greater fraction of the mixture is in its unionised form.
This leads to faster onset, and therefore less delay to the beginning of surgery.
Selected Local Anaesthetics
Lidocaine
Lidocaine is an amide local anaesthetic, with a pKa of 7.9 and protein binding of around 70%. This renders it fast onset (pKa), with a medium duration of action.
It is also a class Ib antiarrhythmic agent.
Bupivacaine
Bupivacaine is another amide local anaesthetic, with a pKa of 8.1 and protein binding of 95%. While slow in onset, it has a substantial duration of action and is therefore useful for prolonged analgesia/anaesthesia. It is commonly employed in regional techniques.
Its S-enantiomer, levobupivacaine, is less cardiotoxic and is often preferred for this reason.
Prilocaine
Yet another amide local anaesthetic, prilocaine is rapid in its onset due to its pKa of 7.8. Its protein binding of 55% gives it a medium duration of action.
Prilocaine can be used in spinal anaesthesia for shorter operations in order to enhance recovery time. Its characteristic side effect is that it can cause methaemoglobinaemia due to generation of o-toluidine during metabolism.
Suggested Reading
Chapter 23. Local Anaesthetics. The Primary FRCA structured oral examination Study Guide 2. 2nd edition. Wijayasiri and McCombe. 2016.
Chapter 11: Local Anaesthetics. Pharmacology for Anaesthesia and Intensive Care. 5th Edition. 2021. Peck and Harris.
BJA article: https://www.bjaed.org/article/S2058-5349(19)30152-0/fulltext